Current Status
On April 25, 2023 the FDA approved tofersen for the treatment of SOD1-ALS under the accelerated approval pathway.
What is tofersen?
Tofersen is an investigational drug, also known as BIIB067, that was developed to treat ALS associated with a mutation in the superoxide dismutase 1 (SOD1) gene.
What is a SOD1 mutation?
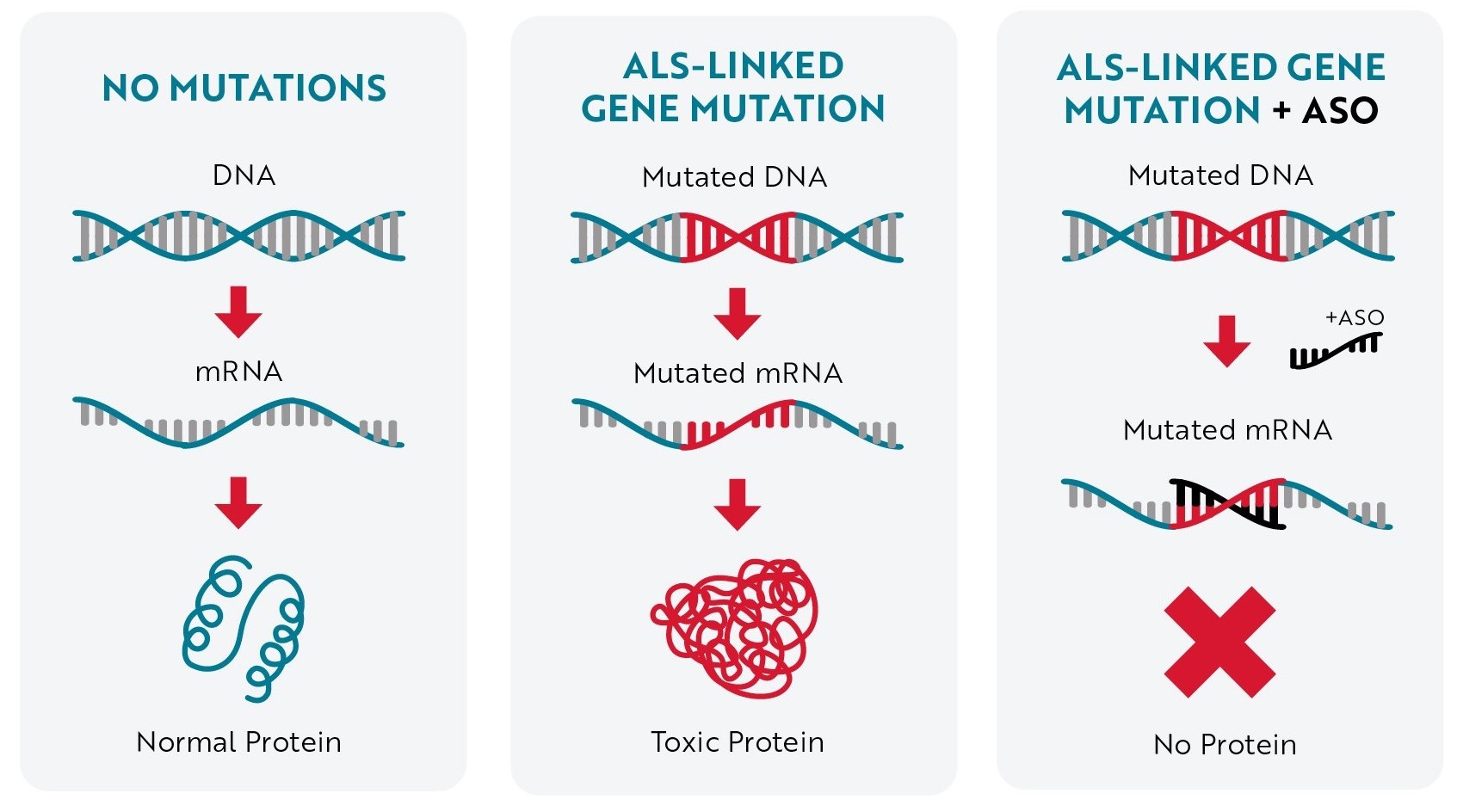
Mutations in the SOD1 gene are the second-most common cause of familial ALS, found in about 10-20% of cases, as well as 1-2% of sporadic ALS cases. The SOD1 gene contains the instructions cells need to produce a protein that is also called SOD1. Healthy SOD1 proteins help break down toxic byproducts produced during normal cell processes. These byproducts must be broken down regularly so they don’t damage cells.
Mutations in the SOD1 gene are thought to cause the protein to misfold and clump up (aggregate) within motor neurons and astrocytes, the types of cells involved in ALS development and progression. These clumps (aggregates) may interfere with healthy cell functions or may cause other necessary proteins to misfold and lose their function, damaging the nervous system and leading to the development of ALS.
How does tofersen work?
Tofersen is an antisense oligonucleotide (ASO), which is a small string of DNA letters (called bases or nucleotides) that are designed to bind to specific molecules of RNA. Tofersen was developed to specifically target the RNA produced from mutated SOD1 genes to stop toxic SOD1 proteins from being made.
What do we know about tofersen’s safety and effectiveness?
A phase 1/2 clinical trial involving a group of 50 people showed that treatment with tofersen was generally safe and lowered levels of the SOD1 protein in cerebral spinal fluid.
To learn more about tofersen’s safety and potential efficacy, researchers then initiated a larger phase 3 clinical trial, known as the VALOR trial, that included 108 adults with ALS caused by mutations in the SOD1 gene. The initial readout of these results, which assessed the effects of 28 weeks of treatment, showed that tofersen did not meet its primary endpoint of slowing the rate of disease progression as measured by the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R), although there were some trends toward benefit that were not statistically significant.
Tofersen was shown to reduce levels of the SOD1 protein in cerebral spinal fluid by 35% as early as eight weeks after participants began receiving the therapy. By 12-16 weeks, tofersen reduced bloodstream levels of NfL, a biomarker of neuron damage and neurodegeneration, by 50%. These biomarker changes were subsequently reflected in functional scale measures. A follow-up study using 12 months of data from the VALOR trial and open label extension showed that earlier initiation of tofersen slowed decline in clinical and respiratory function, strength, and quality of life.
Biogen is currently testing tofersen in a separate phase 3 study, known as the ATLAS Trial. This study is different from the VALOR trial because it involves approximately 150 participants who have specific SOD1 gene mutations but do not have any signs or symptoms of ALS. The researchers are trying to determine whether tofersen can delay the onset of signs or symptoms of ALS and/or slow declines in function once signs or symptoms appear. This trial is projected to be completed in 2027.
How is tofersen administered?
Tofersen is administered through a lumbar puncture. A doctor inserts a thin needle into the space around the spinal cord in the lower back and injects the drug. During the VALOR study, participants received a series of three loading doses followed by monthly intrathecal injections of tofersen.
How is The ALS Association supporting the development of antisense (ASO) technology?
The ALS Association was the first to fund research of ALS-specific ASOs back in 2004 when it was an emerging technology, despite the high risk of the technology not coming to fruition. We subsequently co-funded five additional studies, including preclinical, safety and pharmacodynamic measurements and the first-in-human phase 1 trial assessing safety and target engagement for an early SOD1-targeting antisense drug, ISIS 333611. These studies supported continued investigation of SOD1 as a therapeutic target and led to the discovery of the more potent antisense drug, tofersen.
Over the last two decades, we have committed more than $1.3 million to the development of ASO technology. We are pleased that our focus on translating concepts into therapies has resulted in the advancement of the first-ever gene therapy for ALS as well as potential ASOs for other neurodegenerative diseases, including Huntington’s disease, Alzheimer’s disease, frontotemporal dementia and others.
The success of the antisense drug Spinraza, which is the first FDA-approved treatment for spinal muscular atrophy – a leading genetic cause of death in infants and toddlers, gives us much hope for the future of using antisense therapies to target and treat ALS.
How can people with ALS access tofersen?
To benefit from tofersen, people with ALS must have a mutation in the SOD1 gene. People with ALS and their family members can receive genetic counselling and testing to see if they carry a genetic mutation linked to ALS. Click here for more information about genetic counselling and testing, including free counselling and testing.
While tofersen is considered an “investigational treatment,” people with a mutation in the SOD1 gene are eligible to receive the drug through Biogen’s early access program (EAP) if they live in countries where such programs are permitted by local regulations and future access may be secured. If a clear path forward for tofersen is not established, or if another controlled trial is required by regulators, Biogen may revise or discontinue the EAP. Learn more here.
Learn more about tofersen
Visit Biogen’s website here.
Read past updates from Biogen on tofersen here:
- March 2023: Update on FDA Advisory Committee Meeting on Tofersen for SOD1-ALS
- January 2023: Update on FDA Advisory Committee Meeting
- June 2022: Press Release on 12-Month Results
- October 2021: Community Statement on Topline Results from VALOR
- October 2021: Press Release on Topline VALOR Results
Read past ALS Association content featuring tofersen here:
- March 24, 2023: Why FDA Committee’s Recommendation on Tofersen Matters to Everyone
- March 22, 2023: FDA Committee Unanimously Recommends Accelerated Approval of Tofersen
- March 15, 2023: Tofersen and the FDA Approval Process Webinar
- March 8, 2023: ALS Association Submits Public Comments in FDA Review of Tofersen
- October 17, 2021: Statement on Tofersen results
- December 29, 2020: Biogen Shares Latest Updates on ALS Clinical Trials
- July 16, 2020: Biogen Announces Latest Updates on ALS Clinical Trials During the Pandemic
- May 10, 2019: FAQ: Biogen is Now Enrolling in Phase 3 VALOR Clinical Trial to Test Safety and Efficacy of Tofersen
- May 3, 2019: Biogen to Present Promising Results of Phase 1/2 Trial of Antisense Targeting SOD1
- December 17, 2018: SOD1 Phase I Antisense Trial Shows Promise and C9orf72 Phase I Antisense Trial Begins
