How Biomarkers Are Used
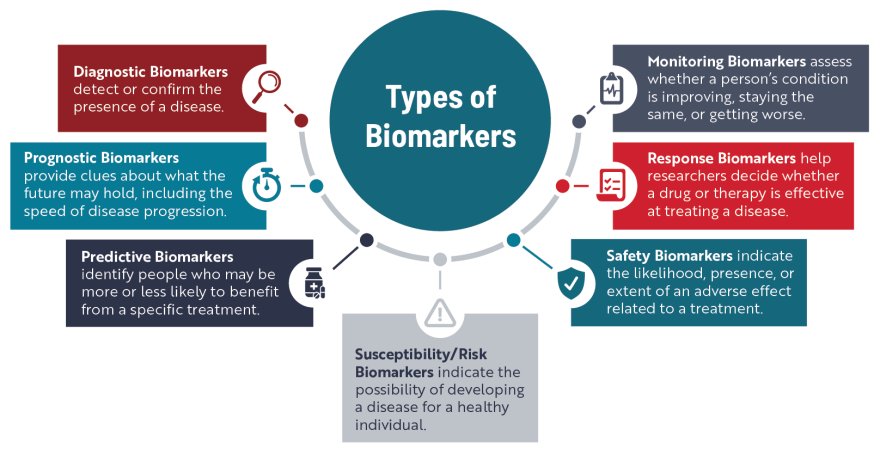
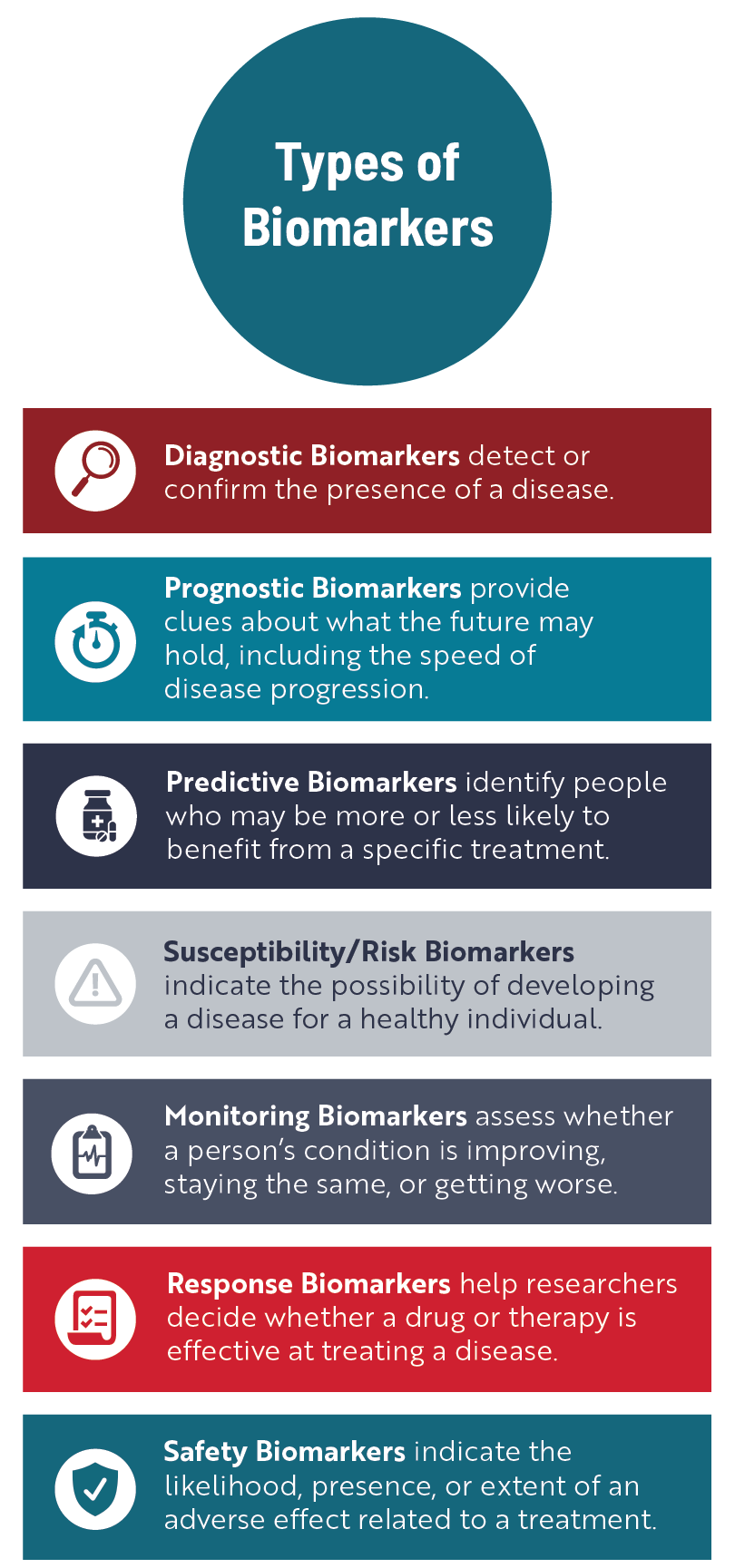
One of the main ways doctors use biomarkers is to better understand what’s happening inside the body to help detect or confirm a specific disease or condition, kind of like a mechanic figuring out what is going wrong inside a car.
Let’s imagine one morning you turn on your car, and the check engine light appears. This light is a sign or “symptom” that something might be wrong. So, you take your car to a mechanic who measures the amount of oil, checks the spark plugs, and runs several other diagnostic tests. Using these “biomarkers,” the mechanic can pinpoint what’s going wrong under the hood. In the same way, doctors test for biomarkers like blood sugar levels to help diagnose diabetes or high numbers of white blood cells to detect an infection.
Health care professionals and researchers also use biomarkers to figure out a person’s risk of developing a disease, track disease progression, better predict the course of a disease, improve clinical trial design, and better assess the efficacy and safety of a treatment.
Biomarkers for ALS
Researchers have been searching for biomarkers specific to ALS for several decades. Although a large body of research exists, there are relatively few validated biomarkers that can be used to identify people who are most at risk of developing ALS, diagnose ALS, monitor how the disease is progressing, or assess the effectiveness of new treatments. Some of the biomarkers making their way more routinely into ALS clinical trials and care are described below.
Genetic Biomarkers
Genetic biomarkers are changes, or mutations, in one or more genes known to cause a disease or are linked to a higher risk of developing a disease. Mutations in more than 40 different genes have been linked to ALS, of which four—C9orf72, SOD1, TARDBP and FUS—account for the disease in up to 70% of people with familial ALS, at least in European populations. The presence of these mutated genes, which are identified through genetic testing, can serve as a susceptibility/risk biomarker for people who do not have any ALS symptoms.
Learn more about ALS-linked genes and mutations
Neurofilament Light Chain (NfL)
Neurofilaments are proteins that are highly specific to neurons and make up about 85% of their protein structure. When neurons are damaged or degenerate, neurofilaments are released into the blood and CSF.
There are several different types of neurofilaments, including heavy chain, medium chain, and light chain. Neurofilament light chain (NfL) is the most studied neurofilament, and NfL levels are significantly elevated in people living with ALS. Because higher than normal levels of NfL are also present in many other neurodegenerative diseases, such as frontotemporal dementia, multiple sclerosis, and Alzheimer’s disease, it is considered “nonspecific” and only has limited potential as a diagnostic biomarker for ALS.
However, research involving people with a mutation in the SOD1 gene but who have no ALS symptoms has shown that NfL levels can start to increase as early as 1 year before symptoms begin, giving it potential as a risk biomarker for SOD1-ALS. Researchers have also linked NfL levels at diagnosis to the speed of ALS progression and survival, suggesting it could be used as a prognostic biomarker.
In 2023, the FDA solidified NfL’s importance as a biomarker in ALS clinical trials with the accelerated approval of tofersen, now known as Qalsody. An FDA advisory committee concluded that a reduction in NfL levels indicated a change in the course of disease for people receiving the treatment, which then could translate over time into benefits such as longer life, increased muscle function, and slower disease progression. Many ALS clinical trials now incorporate NfL measurements into their design to serve as a response biomarker.
Other Emerging Biomarkers
Researchers continue to identify and investigate potential new ALS biomarkers, focusing on substances that can detect and measure disease-causing processes, such as neurodegeneration, inflammation, oxidative stress, excitotoxicity, mitochondrial function, and protein aggregation/buildup.
One area of particular interest is finding biomarkers related to the dysfunction of a protein called TDP-43, which is involved in the development and progression of ALS about 97% of the time. TDP-43 is mainly found in the nucleus of cells, and it is involved in many of the processes used to turn the “blueprints” found in DNA into proteins. In ALS, something goes awry, and TDP-43 leaves the nucleus and can clump together with other proteins, creating aggregates. These aggregates not only keep TDP-43 from performing its important normal functions, but they also block normal cellular processes and cause neurodegeneration.
While TDP-43 aggregates can be found in brain tissue, detecting the disease-causing forms of this protein in the CSF or blood of people living with ALS has been difficult. Researchers continue to develop and refine new strategies to identify and measure the presence of abnormal TDP-43 in neurons, which could be used to help diagnose ALS, monitor disease progression, and eventually generate more precise treatment strategies and better interpretations of clinical trial results.
Explore other biomarkers currently being investigated
Importance of Finding New Biomarkers
Discovering and validating new ALS-specific biomarkers is essential to help diagnose the disease more quickly and accurately and to accelerate the development and availability of new treatments. This is one reason why participating in observational research like natural history studies is so important for people with ALS.
Currently, ALS is considered a “diagnosis of exclusion.” This means people experiencing ALS-like symptoms have to undergo a number of tests designed to rule out other conditions. As a result, it can take roughly a year or more for someone with ALS to receive the correct diagnosis. Having one or more validated diagnostic biomarkers would help clinicians accurately detect ALS earlier, enabling people to start receiving disease-modifying medications at a time when symptoms might be more localized and subtle. Reducing diagnostic delays could also increase the number of people eligible to participate in clinical trials.
Additionally, biomarkers could help researchers design more efficient clinical trials and speed up the overall drug development process, allowing new treatments to reach people with ALS faster. For example, predictive biomarkers could be used to stratify trial participants, so the treatment is tested by individuals who have the highest probability of benefiting. In addition, having sensitive and reliable response biomarkers would make it easier to measure the effectiveness of a new drug or treatment.
Our Support of Biomarker Research
Since 2014, we have supported more than 70 biomarker-focused projects. Currently, this funding is awarded through our Seed Grants and Milton Safenowitz Postdoctoral Fellowship Program, although we also fund biomarker studies as part of larger clinical trials. In addition, through our Partnership Grants, we have supported the development of digital biomarkers and early diagnostic markers for ALS.
Thanks to the generosity of our donors, we continue to make significant investments in building and expanding essential biomarker research infrastructure, such as bio- and data repositories, as well. Biorepositories provide open access to well annotated samples, including blood, CSF, and postmortem tissue, which are essential for biomarker discovery. Equally important are data repositories, such as the Pooled Resource, Open Access ALS Clinical Trials (PRO-ACT) Database, which provide researchers with a large set of well-categorized data collected from individuals over time. This type of data helps researchers more accurately identify trends and changes, especially in rare diseases like ALS.
