Researchers funded by The ALS Association using state-of-the-art technology called an Organ-Chip, which essentially recreates human biology on a microchip, found that the human brain’s tiniest blood vessels can initiate spinal motor neuron development. Studies using this technology to track living tissues on a chip provide ALS researchers a unique way to study the disease processes in ALS and other neurodegenerative disorders.
Drs. Clive Svendsen and Samuel Sances from Cedars-Sinai Board of Governors Regenerative Medicine Institute in Los Angeles published this important work in journal Stem Cell Reports.
The researchers took human skin cell samples and genetically reprogrammed them into induced pluripotent stem cells (iPSCs) that can be turned into many different cell types in the body. Here, they turned iPSCs into spinal motor neurons, the cells that connect to muscle to signal muscle movement and which die in ALS. After creating blood vessel cells as well, spinal motor neurons successfully connected to blood vessels on the Organ-Chip.
We sat down with Dr. Sances, first-author of the study, to learn more about this exciting technology and how it could be applied to understanding the mysteries behind ALS disease pathways.
Congratulations on your paper! Please briefly describe an Organ-Chip.
About the size of a flash drive, Organ-Chips are a new technology that aims to recreate an environment for the cells that make up an organ to live and function outside of the body. Through tiny fluidic chambers, neurons and blood vessel cells grow and interact with each other, an interplay that is critical for neuronal function in the central nervous system.
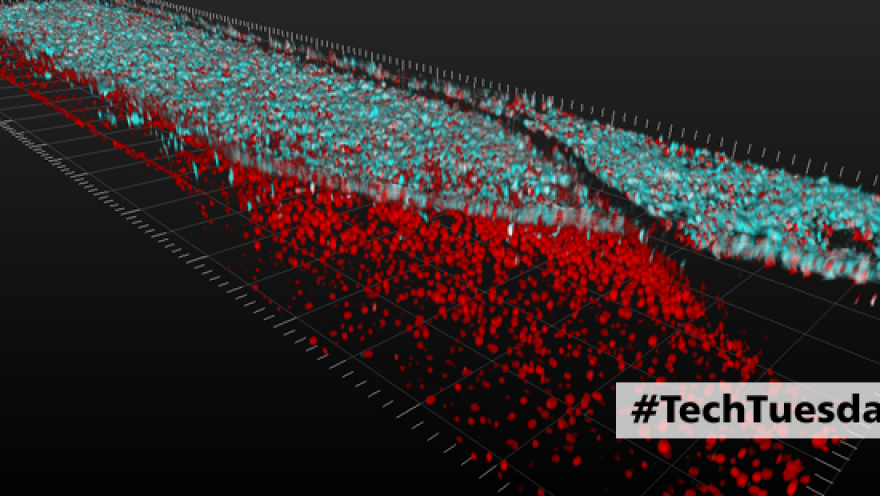
[caption id="attachment_4421" align="alignnone" width="1301"] Layers of spinal motor neuron cells (top, in blue) and capillary cells (bottom, in red) converge inside an Organ-Chip. Neurons and capillary cells interact together along the length of the chip. The image was produced using a confocal microscope; colors were generated by staining with fluorescent antibodies. Image credit: Cedars-Sinai Board of Governors Regenerative Medicine Institute[/caption]
[caption id="attachment_4422" align="alignnone" width="400"] 3D video shows layers of spinal motor neuron cells (top, in blue) and capillary cells (bottom, in red) inside an Organ-Chip. Top-down view shows interconnected network of spinal motor neurons (blue) that interacts with the capillary cells below (in red). The video was produced using a confocal microscope; colors were generated by staining with fluorescent antibodies.
Video credit: Cedars-Sinai Board of Governors Regenerative Medicine Institute[/caption]
[caption id="attachment_4423" align="alignnone" width="400"] Hundreds of spinal motor neurons spontaneously communicate through electrical signals inside an Organ-Chip. Neurons fire individually (flashing dots) and in synchronized bursts (bright waves). The activity was observed using a dye that fluoresces when neurons send an electrical signal.
Video credit: Cedars-Sinai Board of Governors Regenerative Medicine Institute[/caption]
What are the most important findings of your paper?
We created a Spinal Cord-Chip to study the effects of lab-generated blood vessel cells on living spinal motor neurons that had also been generated from human patients’ stem cells. We found that the vessel cells impart molecular signals that enhance spinal motor neuron development and made them more like their counterparts in then human embryo. This sheds light on both how our spinal cord develops and sets the stage for improved ALS modeling and therapeutic discovery in vitro.
“Until now, people thought these blood vessels just delivered nutrients and oxygen, removed waste and adjusted blood flow. We showed that beyond plumbing, they are genetically communicating with the neurons.” – Dr. Sances
How can Organ-Chip be applied to ALS research now and in the future?
The Spinal-Cord Chip is now being used in a large study that includes ALS-patients in search of new biomarkers and disease targets for therapy development. Importantly, the intact nature of the blood vessel compartment could enable rapid drug development by studying the effects of delivering experimental therapeutics through blood vessels into neural tissue.
Read the press release here.
Read the open access paper here.
Paper citation
Human iPSC-Derived Endothelial Cells and Microengineered Organ-Chip Enhance Neuronal Development. Sances, S., et al. Stem Cell Reports. Published online: March 22, 2018.
DOI: https://doi.org/10.1016/j.stemcr.2018.02.012

Join the conversation. Please comment below.