UPDATE – APRIL 2024: Relyvrio was voluntarily withdrawn from the market by Amylyx following a phase 3 trial that failed to show it was effective. The ALS Association stands by its decision to push for early approval of Relyvrio given the promising phase 2 trial data and the safety of the treatment. At the time, we said that if it turns out to be ineffective, at worst, people living with ALS would have taken an ineffective therapy without risk of harm. If it was indeed effective, delaying access would have meant that people living with ALS would have lost two years of being able to take a life-extending therapy. In the interests of transparency and education, we are leaving this information up for future reference. People living with ALS need life-saving treatments and we are working as urgently as possible to advance the many more potential treatments in clinical trials.
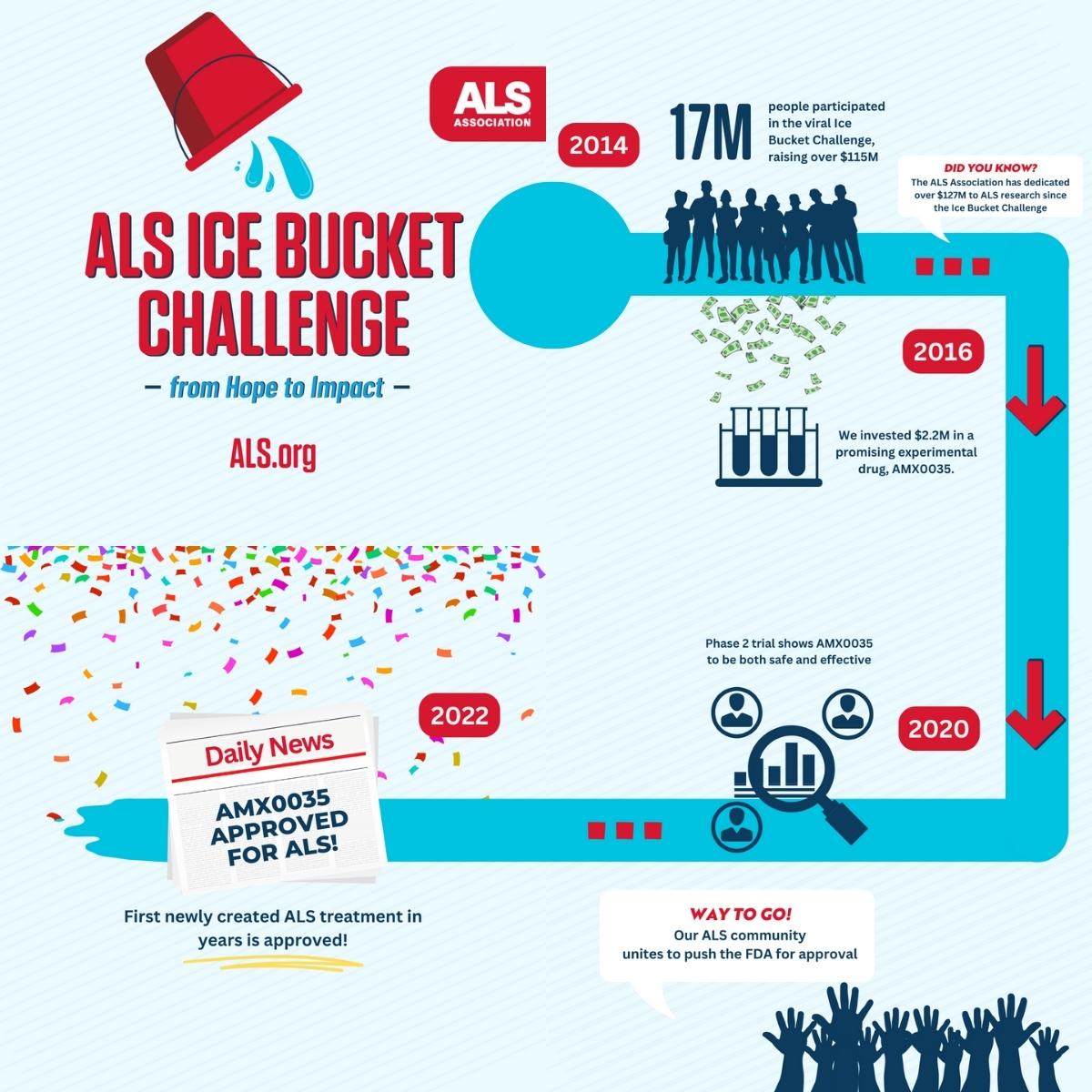
In the summer of 2014, millions of us took part in the ALS Ice Bucket Challenge. Celebrities, friends and family members all dumped ice on their heads to help raise awareness and funds for ALS. Thanks to everyone who donated, new ALS genes were discovered, new global research projects were funded and care services were expanded to help more people with ALS.
One of the potential treatments that the ALS Association funded was AMX0035, which was first imagined by two Brown University students in a dorm room the year before the Ice Bucket Challenge.
The ALS Association spent over $2 million helping fund the development and clinical trial of AMX0035. When the results of that trial showed it was safe and effective in treating ALS, the ALS Association led an advocacy campaign to push the FDA to approve the drug. After two years of advocacy, the FDA finally approved AMX0035.
“This is a victory for the entire ALS community, which came together to advocate for early approval of AMX0035. We still have a lot of work to do to cure ALS, but this new treatment is a significant step in that fight,” ALS Association President and CEO Calaneet Balas said in a statement.
In September of 2020, The ALS Association and I AM ALS submitted over 50,000 signatures to the FDA calling on the agency to approve AMX0035. In subsequent months, the Association held multiple meetings with FDA officials, including a public We Can’t Wait Action Meeting in May of 2021, so members of the ALS community could speak directly to FDA officials. The Association also provided scientific and regulatory guidance and in 2022, drove over 14,000 individual emails to the FDA urging approval.
Steve Kowalski, who was diagnosed with ALS in 2017, testified twice before the FDA’s advisory committee pleading for quick approval.
“This is a positive step forward on that path to making ALS a livable disease until we find a cure. It means the possibility of increasing precious time with friends and family, greater independence and improving the quality of life for people living with ALS today,” he said of the decision.
Sunny Brous, a dedicated advocate for swift approval of safe and effective treatments, also celebrated the approval of AMX0035.
"When I took the Ice Bucket Challenge, I never imagined I would be diagnosed with ALS a few months later at the age of 27. Approval of AMX0035 is a shared victory for everyone living with ALS, and a tribute to those we’ve lost. We can agree this disease is different for everyone and approval means a new line of defense while moving us one step closer to making ALS a livable disease,” she said.
Before AMX0035 will be made available, payers like Medicare and private insurance companies have to make determinations on whether and how they will cover the drug.
For more on how insurance coverage decisions are made, listen to this discussion on Connecting ALS.
This is a hugely meaningful win for the community. Getting effective new drugs approved and made available as quickly as possible is key to making ALS a livable disease. Thanks to everyone who worked tirelessly to make this happen, and to everyone who took the ALS Ice Bucket Challenge.


Comments
Great news . Every step in the right direction is a positive step to a cure for this terrible disease
#SlowStopReversePrevent
Happy lost my son in 2019 of this disease. Now that is approved. It should be free to anyone living with ALS. It shouldn't be a price on your life. Some people may not be able to afford it. This disease is dreadful. Make to where everyone with any kind of insurance can afford it. Be Bless
I am so happy when FDA approved AMX0035. I wonder when this drug is availaible and whether I have to pay for the drug or not.
when will amx00035 be available to the public???????????!
Hi Dave. The FDA has approved AMX0035 (now known as RELYVRIO). You will need a prescription from your doctor in order to access it. A prescription guide is available here: https://www.amylyx.com/product
Join the conversation. Please comment below.