Each year on February 11, the world celebrates International Day of Women and Girls in Science Day, a day to remind us of the critical role women and girls play in science and technology communities and the importance of bringing fresh perspectives, talents and creativity to the field of STEM.
This year we’re shining a spotlight on Dr. Allison Ebert, Ph.D., Leadership Team Member at The ALS Association Wisconsin Chapter & professor at the Medical College of Wisconsin. Dr. Ebert breaks down the challenges of finding effective therapies for people living with ALS and the important work happening in her lab.
My name is Allison Ebert, and I’m an Associate Professor at the Medical College of Wisconsin (MCW). I have a Ph.D. in neuroscience from Northwestern University, and I did postdoctoral training in stem cell biology at the University of Wisconsin-Madison with Dr. Clive Svendsen. I started my independent faculty career at MCW in January 2011, and my lab focuses on uncovering mechanisms leading to motor neuron loss in ALS and other related motor neuron diseases.
There are several challenges to finding effective therapies for ALS, but a really big one is that we need a better grasp on why motor neurons get sick.
The nervous system is incredibly complex, and there are several different types of cells that interact and communicate with each other; we need to know which cells are “talking” with whom and what they are “saying”. My lab is trying to tackle this question by testing how chemical signals produced from astrocytes and microglia, two very important cell types found in the brain and spinal cord, lead to nerve cell loss.
Astrocytes play a role in providing nutrients and maintaining a healthy environment around the nerve cells, and microglia are the resident immune cells in the nervous system. However, in disease settings, astrocytes and microglia stop performing their required tasks and essentially create chaos. Another big issue is that all ALS patients are unique individuals, so creating experimental models that replicate ALS disease features across patients can be very difficult.
Many studies have used genetically engineered mice to study how ALS progresses in a living creature. These studies have been incredibly valuable, but we know that mice aren’t humans.
My lab uses a stem cell system, called induced pluripotent stem cells (iPSCs), that are generated from ALS patients.
iPSCs are created in a laboratory from a patient’s skin cell or blood cell. We use genetic manipulation to convert the donor cell into a blank-slate cell that can then be given specific chemical cues in a petri dish to make the astrocytes, microglia, and motor neurons that we are interested in studying.
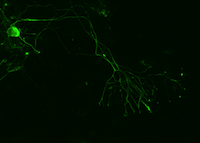
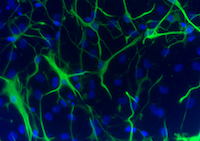
These pictures show microscope images of a motor neuron (left) and astrocytes (right) that were produced from iPSCs (we colored them green to be able to see them under the microscope). iPSCs allow us to create a human “disease-in-a-dish”.
In order to understand and eventually stop the chaos occurring in the brain and spinal cord during ALS, we grow motor neurons, astrocytes, and microglia from ALS iPSCs in petri dishes and monitor what chemical signals are being produced and how those chemical signals impact motor neuron health and function. We have recently found that motor neurons from a sporadic ALS patient’s iPSCs, but not from a healthy unaffected person, show accumulation of neurofilament protein, a key hallmark of ALS pathology observed in patient spinal cord.
We found that treating healthy motor neurons with chemical signals released from ALS astrocytes causes neurofilament accumulation.
However, if we treat the ALS astrocytes with chemical signals released from healthy microglia before we treat the motor neurons, we are able to reduce the neurofilament accumulation. Excitingly, these results suggest that healthy microglia produce signals that can limit the toxic effect of ALS astrocytes. We first need to repeat these studies with more samples, but then we aim to figure out what specific signals the microglia are producing and how those signals are able to reduce the astrocyte toxicity to motor neurons. Ultimately, we plan to use this information to develop more effective therapeutic interventions.
This is a very exciting time for my lab as we pursue these experiments, and I look forward to seeing what we can discover.
Special thanks to Dr. Ebert and the team at The ALS Association Wisconsin Chapter for allowing us to share her inspiring work in the field of ALS research. To continue to learn more about ALS and follow stories about people in the community, follow our blog at ALS.org/blog.
About the Author:
Dr. Allison Ebert, Ph.D., is Associate Professor of Cell Biology, Neurobiology and Anatomy and Director of the Neuroscience Doctoral Program at the Medical College of Wisconsin (MCW).

Join the conversation. Please comment below.